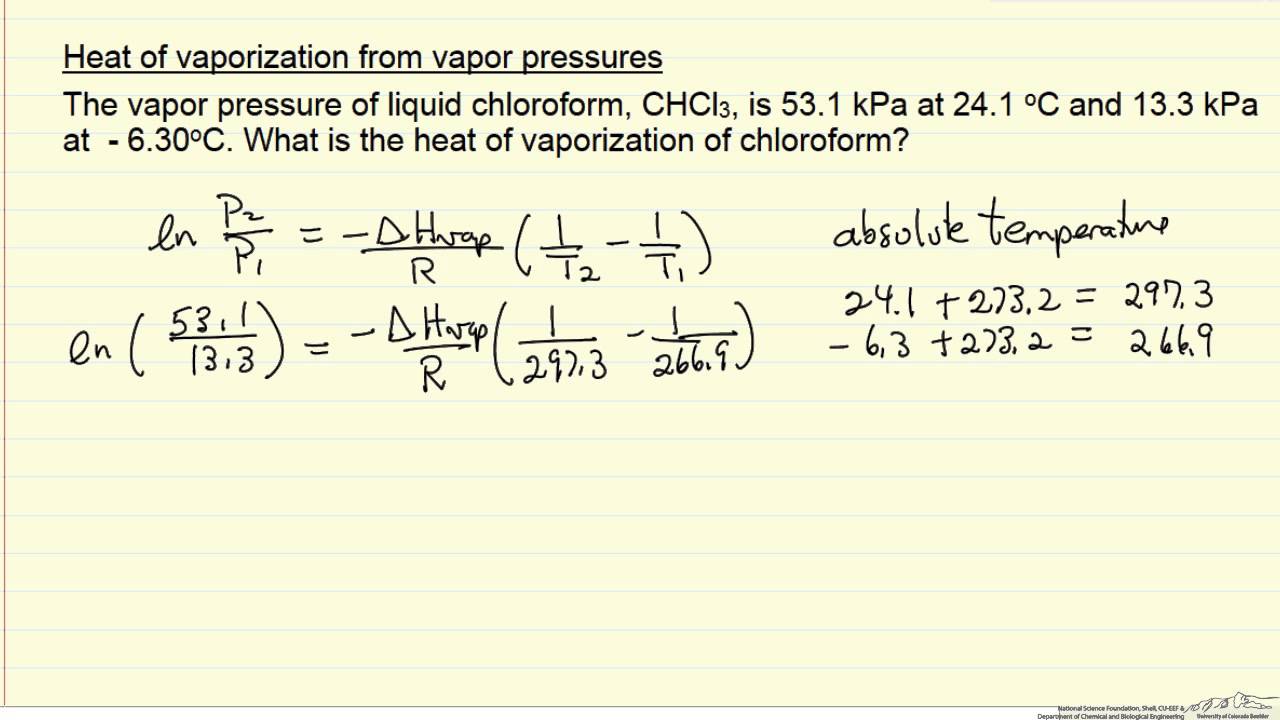
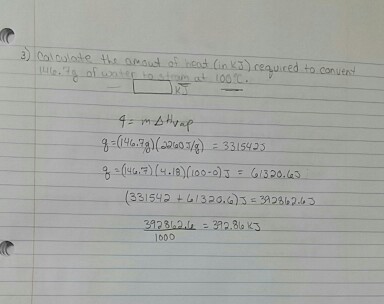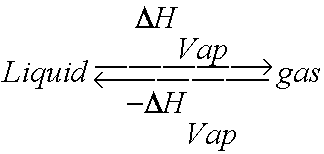thermodynamics - $\Delta \bar{h}_{vap}$ and $\Delta \bar{s}_{vap}$ from vapor pressure vs. temperature data - Chemistry Stack Exchange

For a substance with the value of DeltaH(vap) and DeltaS(vap) given below, what is its normal boiling point in .^(@)C (DeltaH(vap)=59.0 kJ mol^(-1), DeltaS(vap)=93.65 Jmol^(-1))
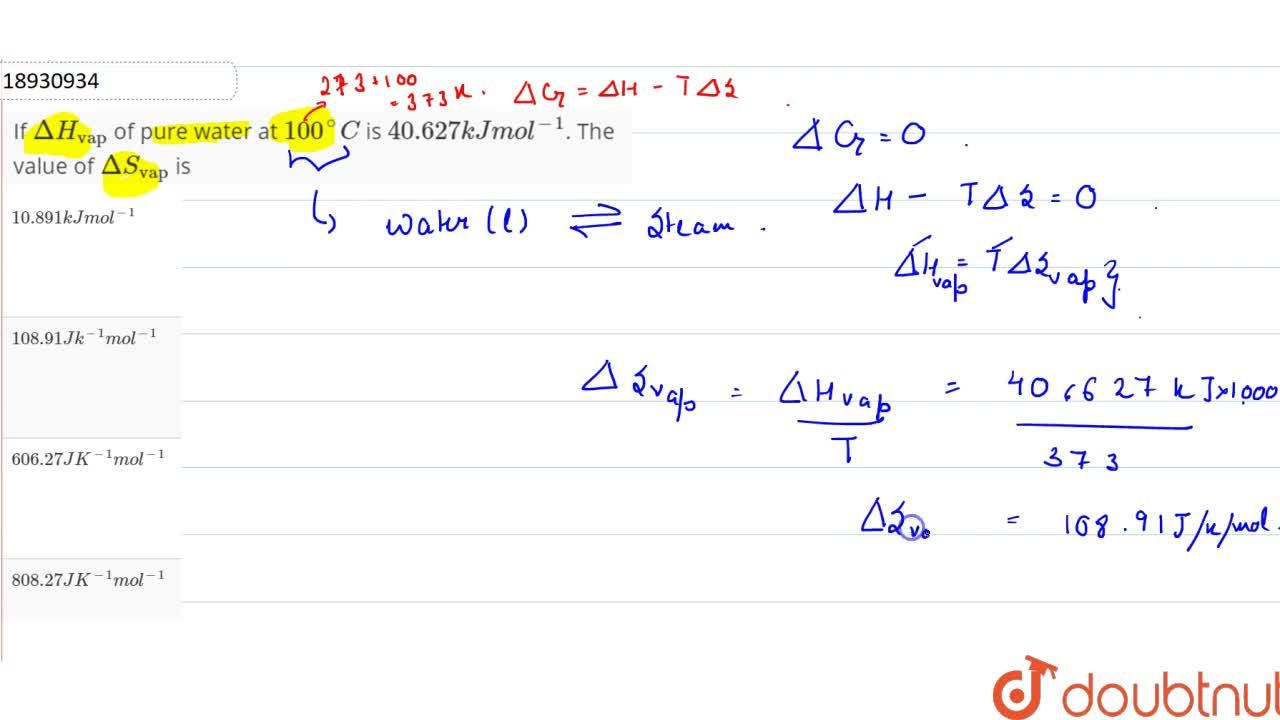
Standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol^-1. - Sarthaks eConnect | Largest Online Education Community
88. The values for delta H vap.and delta S vap. for ethanol are respectively 38.594 kJ/mol and 109.8 J/K. What will be the boiling point of ethanol ?

SOLVED:What is the ΔH vap ^∘ of a liquid that has a vapor pressure of 621 torr at 85.2^∘ C and a boiling point of 95.6^∘ C at 1 atm ?

SOLVED:For mercury, the enthalpy of vaporization is 58.51 kJ/mol and the entropy of vaporization is 92.92 J/K ? mol. What is the normal boiling point of mercury?

SOLVED: The Clausis-Clapeyron equation relates vapor pressure; enthalpy of vaporization, and temperature: AHL In p The equation can als0 be expressed in two-point form; AH, Decide which form to use,and solve the